June 2025

Discoveries, trends, and discussions in medical diagnostics
1) June 27 is National HIV testing day
2) Detect dangerous NPS combinations with confidence
3) Give patients the power of prevention with PrEP
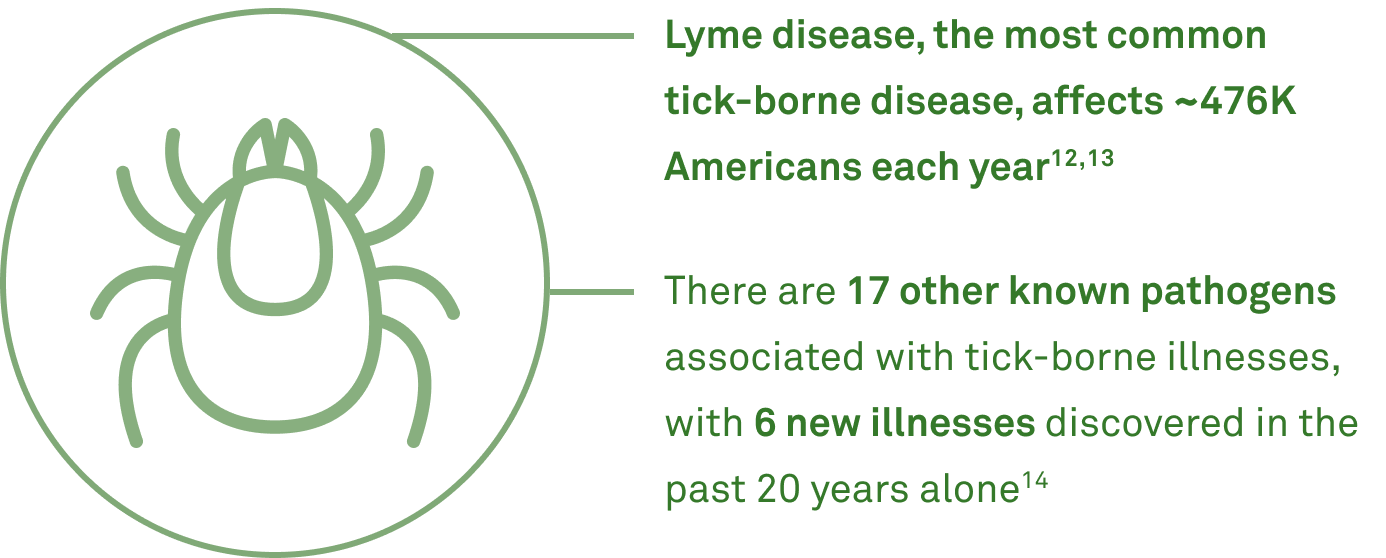
4) Make timely tick-borne disease diagnoses
5) Understand the gut-health connection
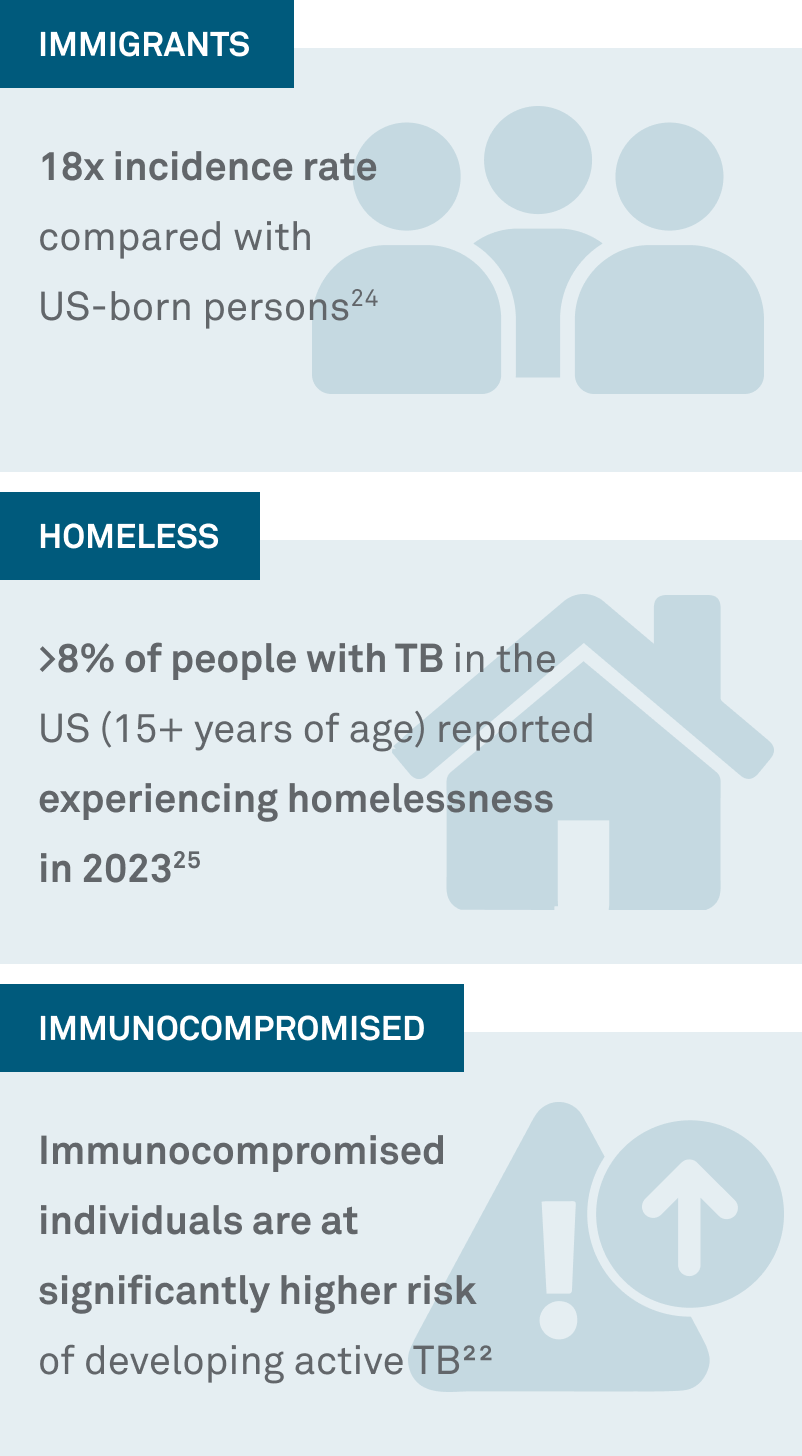
6) Test the right patients to help stem the rising tide of TB
7) HbA1c testing is covered by Medicare to help improve diabetes screening
|
|
June 27 is National HIV testing day |
|
|
June 27 is National HIV testing day |
|
|
June 27 is National HIV testing day |
June 27 is National HIV testing day |
Each year in the US, National HIV testing day is observed on June 27 to raise awareness about the importance of testing for HIV and getting an early diagnosis.
HIV remains a persistent problem in the US:


The CDC recommends2:
|
|
Everyone between the ages of 13 and 64 should get tested for HIV at least once |
|
|
People with certain risk factors should get tested more often |
With assays for all stages of the HIV patient journey, Quest can help you support your patients throughout their care continuum, from prevention and screening to monitoring treatments.

|
Better outcomes |
Better outcomes |
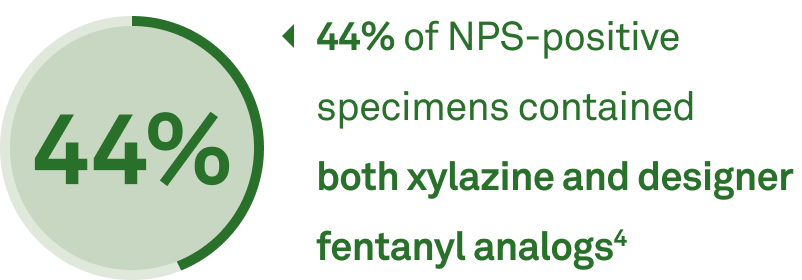
Detect dangerous NPS combinations with confidence
- Designer fentanyl analogs ie, carfentanil, chlorofentanyl
- Xylazine and other illicit additives ie, tianeptine
- Designer benzodiazepines ie, BZS, clonazolam
- Designer opioids ie, nitazenes
- Designer stimulants ie, fluorexetamine
- Synthetic cannabinoids ie, ADB-5Br-PINACA
Give patients the power of prevention with PrEP
- HIV-positive sexual or injection partner
- Bacterial sexually transmitted infection in the past 6 months
- History of inconsistent or no condom use with sexual partner(s)
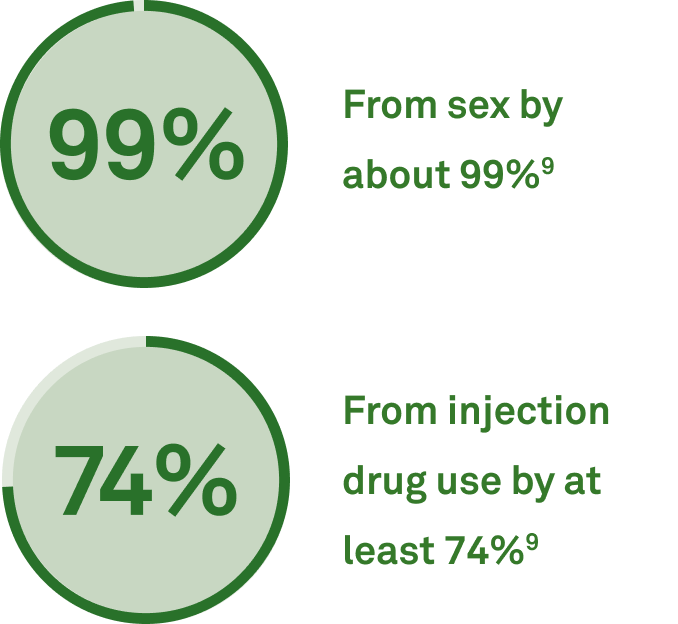
Make timely tick-borne disease diagnoses
- 50% of patients suffering from post-treatment Lyme disease had coinfections15
- 30% had 3 or more simultaneous infections15

|
Better experiences |
Better experiences |
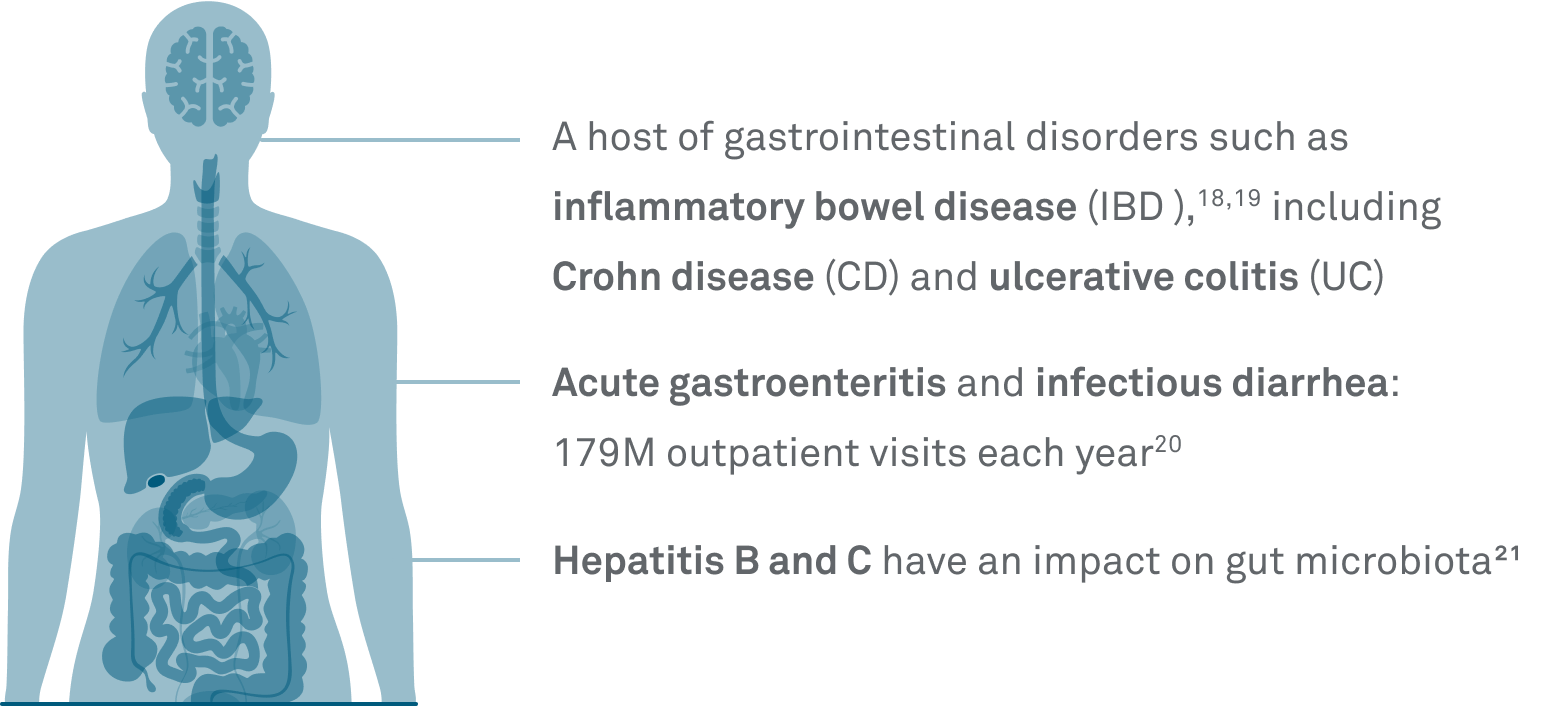
Understand the gut-health connection
Test the right patients to help stem the rising tide of TB
|
|
>23% of TB cases occurred in people with diabetes22 |
|
|
>4% of people with TB are coinfected with HIV27 |
|
|
It’s 9x more likely for people with certain cancers to develop active TB compared to those without cancer28 |

|
Removing traditional barriers to testing |

|
Removing traditional barriers to testing |
Removing traditional barriers to testing |
HbA1c testing is covered by Medicare to help improve diabetes screening
|
|
Hypertension |
|
Dyslipidemia |
|
Obesity |
|
History of hyperglycemia |
- Is 65 years of age or older
- Is overweight
- Has parents or siblings with a history of diabetes
- Has a history of gestational diabetes or delivered a baby weighing more than 9 lbs
Achieving the Quadruple Aim in your practice
About Diagnostics Dialogue
- HIV.gov. US statistics. February 21, 2025. Accessed May 5, 2025. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics
- CDC. Getting tested for HIV. February 11, 2025. https://www.cdc.gov/hiv/testing/index.html
- Quest Diagnostics Health Trends. Drug misuse in America 2023: the growing crisis of novel psychoactive substances. December 23, 2024. Accessed February 12, 2024. https://mma.prnewswire.com/media/2303443/Quest_Diagnostics_2023_HT_DM_Report.pdf?p=original
- Quest Diagnostics NPS report, 2025.
- Kariisa M, O’Donnell J, Kumar S, et al. Illicitly manufactured fentanyl–involved overdose deaths with detected xylazine— US, January 2019–June 2022. MMWR Morb Mortal Wkly Rep 2023;72:721-727. doi:10.15585/mmwr.mm7226a4
- Iwersen-Bergmann S, Lehmann S, Heinemann A, et al. Mass poisoning with NPS: 2C-E and Bromo-DragonFly. Int J Legal Med. 2019;133(1):123-129. doi:10.1007/s00414-018-1882-9
- Peacock A, Bruno R, Gisev N, et al. New psychoactive substances: challenges for drug surveillance, control, and public health responses. Lancet. 2019;394(10209):1668-1684. doi:10.1016/s0140-6736(19)32231-7
- Office of Infectious Disease and HIV/AIDS Policy, HHS. Ready, Set, PrEP. HIV.gov. Updated March 18, 2022. Accessed October 5, 2023. https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/prep-program/
- CDC. Pre-Exposure Prophylaxis (PrEP). Updated July 5, 2022. Accessed October 5, 2023. https://www.cdc.gov/hiv/risk/prep/index.html
- US Public Health Service, CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2021 update – a clinical practice guideline. Accessed October 5, 2023. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Winny A. Tickborne diseases are on the rise—here’s what to know. Johns Hopkins. June 21, 2023. Accessed March 19, 2024. https://publichealth.jhu.edu/2023/lyme-disease-isnt-the-only-tickborne-disease-to-watch
- Schwartz AM, Kugeler KJ, Nelson CA, et al. Use of commercial claims data for evaluating trends in Lyme disease diagnoses, United States, 2010-2018. Emerg Infect Dis. 2021;27(2):499-507. doi:10.3201/eid2702.202728
- Kugeler KJ, Schwartz AM, Delorey M, et al. Estimating the frequency of Lyme disease diagnoses—United States, 2010-2018. Emerg Infect Dis. 2021;27(2):616-619. doi:10.3201/eid2702.202731
- US Dept of Health and Human Services. Tick-borne disease working group—2018 report to Congress. August 10, 2017. Accessed March 19, 2024. https://www.hhs.gov/sites/default/files/tbdwg-report-to-congress-2018.pdf
- Johnson L, Wilcox S, Mankoff J, et al. Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey. PeerJ. 2014;2:e322. doi:10.7717/peerj.322
- CDC. Testing and diagnosis for Lyme disease. Accessed February 14, 2025. https://www.cdc.gov/lyme/diagnosis-testing/index.html
- Wiertsema SP, van Bergenhenegouwen J, Garssen J, et al. The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in optimizing treatment strategies. Nutrients. 2021;13(3):886. doi:10.3390/nu13030886
- Ni J, Wu G, Albenberg L, et al. Gut microbiota and IBD: causation or correlation?. Nat Rev Gastroenterol Hepatol. 2017;14(10):573-584. doi:10.1038/nrgastro.2017.88
- Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145:16-27. doi:10.1016/j.jaci.2019.11.003
- Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):1963-1973. doi:10.1093/cid/cix669
- Neag MA, Mitre AO, Catinean A, Buzoianu AD. Overview of the microbiota in the gut-liver axis in viral B and C hepatitis. World J Gastroenterol. 2021;27(43):7446-7461. doi:10.3748/wjg.v27.i43.7446 5
- CDC. Reported tuberculosis in the US, 2023. About the data. November 5, 2024. Accessed March 19, 2025. https://www.cdc.gov/tb-surveillance-report-2023/summary/index.html
- US Preventive Services Task Force. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2023;329(17):1487-1494. doi:10.1001/jama.2023.4899
- CDC. Health disparities in tuberculosis. January 31, 2025. Accessed March 19, 2025. https://www.cdc.gov/tb/health-equity/index.html
- CDC. TB risk and people experiencing homelessness. October 30, 2024. Accessed March 19, 2025. https://www.cdc.gov/tb/risk-factors/homelessness.html
- CDC. Testing for tuberculosis. June 17, 2024. Accessed April 8, 2024. https://www.cdc.gov/tb/testing/index.html
- CDC. Reported tuberculosis in the US, 2022. Updated November 15, 2023. Accessed June 25, 2024. https://www.cdc.gov/tb/statistics/reports/2022/table17.htm
- Cheng MP, Abou Chakra CN, Yansouni CP, et al. Risk of active tuberculosis in patients with cancer: A systematic review and meta-analysis. Clin Infect Dis. 2017;64(5):635-644. doi:10.1093/cid/ciw838
- CDC. National diabetes statistics report. Updated May 15, 2027. Accessed May 28, 2025. https://www.cdc.gov/diabetes/php/data-research/index.html
- Centers for Medicare and Medicaid Services (CMS). Your Medicare coverage: diabetes screening. Accessed December 12, 2023. https://www.medicare.gov/coverage/diabetes-screenings